When you’re drowning in dropsIntervene early and rise above with iDose TR
A paradigm-changing technology
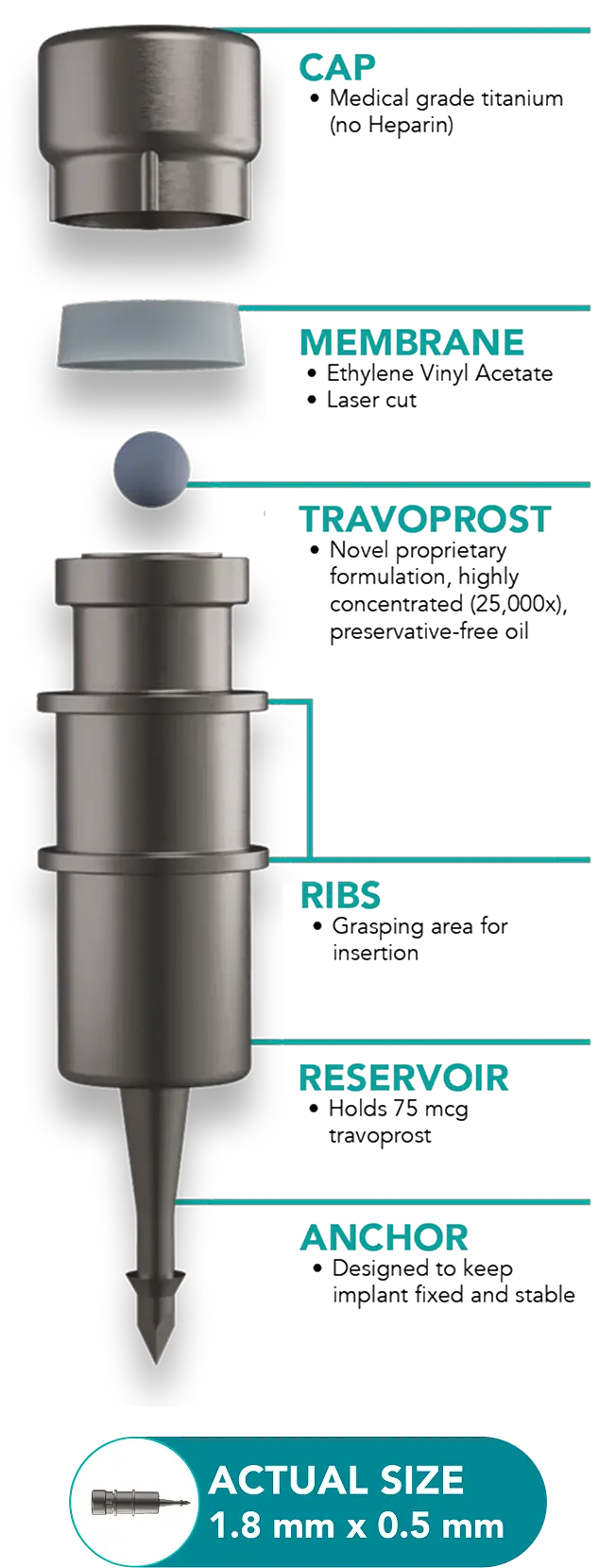
iDose TR is a procedural pharmaceutical that delivers prostaglandin analog therapy for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.1
24/7 adherence
First-of-its-kind anchored implant designed to deliver continuous drug therapy1,2
Handcrafted
Each iDose TR is individually fashioned through micro-scale design capabilities using biocompatible materials1
Controlled delivery
Proprietary membrane with nanoporous material to control drug elution while delivering therapy1,3
Designed to help reduce the flood of issues caused by drops1-6

Replace a flood of drops with iDose TR2
Actual size of implant is 1.8mm x 0.5 mm2 – immensely smaller than a single eye drop.
iDOSE TR EFFICACY
GLAUKOS SUPPORT
Ready to hear more?
Contact a representative to request more info.
References: 1. iDose TR (travoprost intracameral implant) 75 mcg Prescribing Information. Glaukos Corporation. 2023. 2. Berdahl JP, Sarkisian SR Jr, Ang RE, et al. Efficacy and safety of the travoprost intraocular implant in reducing topical IOP‐lowering medication burden in patients with open‐angle glaucoma or ocular hypertension. Drugs. 2024;84(1):83-97. 3. iDose TR Phase 3 Clinical Trials, data on file, Glaukos Corporation. 4. Teymoorian S, Kaur J. Travoprost intracameral implant in eyes with glaucoma or ocular hypertension: early short-term real-world outcomes. Clin Ophthalmol. 2025;19:157-166. 5. Economic burden worsens for glaucoma patients. Review of Optometry. Published March 24, 2021. Accessed August 7, 2025. https://www.reviewofoptometry.com/article/econcmic-burden-worsens-for-glaucoma-patients 6. Nordstrom BL, Friedman DS, Mozaffair E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598-606.