To rise above the flood of dropsiDose TR delivers as designed
In 2 pivotal trials, iDose TR achieved the prespecified primary efficacy endpoint (noninferiority to topical timolol through 3 months).1
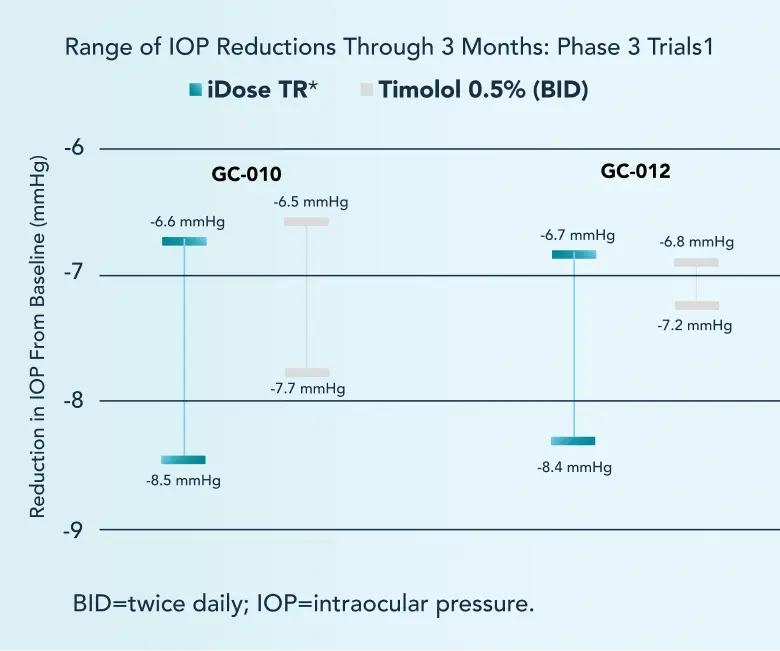
Noninferiority of iDose TR to timolol was established if the upper limit of the 2-sided 95% confidence interval for the difference in the mean of change from baseline in IOP was <1.5 mmHg at each of the 6 post-baseline timepoints and was <1 mmHg at 3 or more of the 6 post-baseline timepoints.2,3
Trial design: iDose TR was evaluated for efficacy and safety in patients with OHT and OAG. Two identically designed, Phase 3, parallel-group, double-masked, randomized, prospective, sham-controlled trials compared iDose TR with topical timolol (0.5%) BID. The primary endpoint was the change in diurnal IOP (vs timolol) through 3 months post implantation. Patients aged ≥18 years with OAG (POAG, PXG, or PG) or OHT, with a C/D ratio of ≥0.8, and with a BSCVA of 20/80 or better were included in the study. Change was assessed from a time-matched baseline in IOP from 8 AM and 10 AM at Day 10, Week 6, and Month 3. Mean baseline IOP was 24.6 mmHg.1-3
BSCVA=best spectacle-corrected visual acuity; C/D=cup-to-disc; OAG=open-angle glaucoma; OHT=ocular hypertension; PG=pigmentary glaucoma; POAG=primary open-angle glaucoma; PXG=pseudoexpoliative glaucoma.
Enduring control at 12 months3,*
81%
of iDose TR patients were completely free of IOP-lowering topical medications (23% were originally on 2+ medications pre trial)
ABOUT iDOSE TR
iDOSE TR SAFETY
Ready to hear more?
Contact a representative to request more info.
References: 1. iDose TR (travoprost intracameral implant) 75 mcg Prescribing Information. Glaukos Corporation. 2023. 2. Sarkisian SR Jr, Ang RE, Lee AM, et al; GC-010 Travoprost Intraocular Implant Investigators. Phase 3 randomized clinical trial of the safety and efficacy of travoprost intraocular implant in patients with open-angle glaucoma or ocular hypertension. Ophthalmology. 2024;131(9):1021-1032. 3. Sarkisian SR, Ang RE, Lee AM, et al. Travoprost intracameral implant for open-angle glaucoma or ocular hypertension: 12-month results of a randomized, double-masked trial. Ophthalmol Ther. 2024;13(4):995-1014. 4. Berdahl JP, Sarkisian SR Jr, Ang RE, et al. Efficacy and safety of the travoprost intraocular implant in reducing topical IOP‐lowering medication burden in patients with open‐angle glaucoma or ocular hypertension. Drugs. 2024;84(1):83-97.